Our Research
Our research is guided by two overarching questions:
-
What aspects of adolescent development render an individual susceptible to neuropsychiatric disease?
-
How do dopaminergic microcircuit mechanisms governing information encoding evolve across the lifespan in normal and disease states?
Adolescence represents a precarious period of vulnerability, during which behavioral and neurobiological traits are predictive of later neuropsychiatric diagnoses across a wide range of disease states, from mood and substance use disorders to schizophrenia and eating disorders. In addition to the emergence of neurobiological markers, prodromal behavioral manifestations of these diseases often first appear during early adolescence. Because of the wide consensus that the mesolimbic dopamine system is central to the etiology of many of these disorders, our lab focuses on defining the developmental trajectory of microcircuit control of dopamine dynamics, especially during adolescence, that leaves an individual vulnerable to maladaptive decision-making characteristic of the many forms of neuropsychiatric disease.
Research Techniques
Cell Culture
While it's important to understand how neurons function within microcircuits in situ, dissecting the functional properties of microcircuits also requires the ability to deconstruct them to their core parts. In combination with the other techniques listed below, we sometimes use cell culture to help us answer basic questions about the development of microcircuits.

Behavior
Both on its own or when combined with other techniques listed here, behavior is a powerful technique for understanding how biological properties of the brain manifest in vivo. Our lab uses mouse models to understand many aspects of communication, motivation, learning and affect.
Immunohistochemistry and Western Blotting
Both of these techniques are essential techniques for studying the presence, distribution, and abundance of specific proteins in the brain. IHC allows us to visualize where proteins are located within brain tissue using labeled antibodies, helping to map neural circuits, identify cell types, and observe changes in protein expression in response to experimental manipulation. Western blotting, on the other hand, enables quantitative analysis of protein levels in brain tissue samples. Together, these techniques give complementary insights—spatial information from IHC and quantitative data from western blotting—critical for understanding the molecular mechanisms underlying brain function and dysfunction.
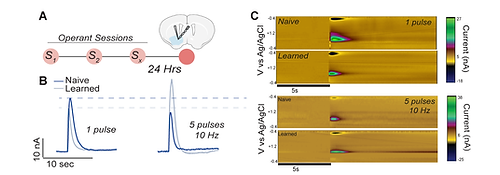
Fast-scan cyclic voltammetry (FSCV) uses carbon-fiber microelectrodes and rapid voltage sweeps to detect neurotransmitters like dopamine on a subsecond timescale, offering high temporal and spatial resolution. In slices, this allows our lab to monitor neurotransmitter dynamics in response to stimulation and pharmacological manipulation. Unlike traditional biochemical assays, FSCV provides insights into the timing and modulation of neurotransmission, making it a unique technique. We can also combine this with other manipulations to understand how genes and experiences modify properties of presynaptic release within dopaminergic microcircuits.
Fast-Scan Cyclic Voltammetry

Optical Slice Imaging
Optical slice imaging enables the vast world of biosensors, from genetically encoded sensors for calcium and neurotransmitters like dopamine, to voltage sensors, to even cell permeable dyes. Used in conjunction with the other techniques listed here, we can parse aspects of intact microcircuits and ask questions about how they function at baseline as well as after different experimental manipulations.